|
Tiger Software's Biotech Series Continued
- Part 2



AMGEN's Take-Off Phase: 1990
When The Tide Goes Out, You Can See the Rocks
 When The Stock Market Falls, The Very Strongest Stocks Stand Out. When The Stock Market Falls, The Very Strongest Stocks Stand Out.
Tiger's Accumulation Index Made Us A Lot of Money Here.
We said last time that Biotechs as a group move in sudden sweeps upward that last a year
and half to two years. The
advances are then followed by seven or eight years of stagnancy.
When the Biotechs as a group do
start a move upwards by making a breakout new high above
a series of previous peaks at about
the same level, it will pay to be in the right biotechs.
Amgen's success is instructive.
And it is a stock we made a lot of money in for ourselves and
for our subscribers back in 1990
and 1991.
Formed in 1980 by some scientists and venture capitalists with a $19 million private
placement, Amgen (AMGN) is surely one of
the very best performing biotechs of all time. It is one
of the only biotech companies to grow
from being a drug development company into a pharmaceutical
manufacturer. It owes its success
to two gene spliced drugs, Neupogen
and Epogen.
Additional
public offerings were required in 1983,
1986 and 1987.
The first
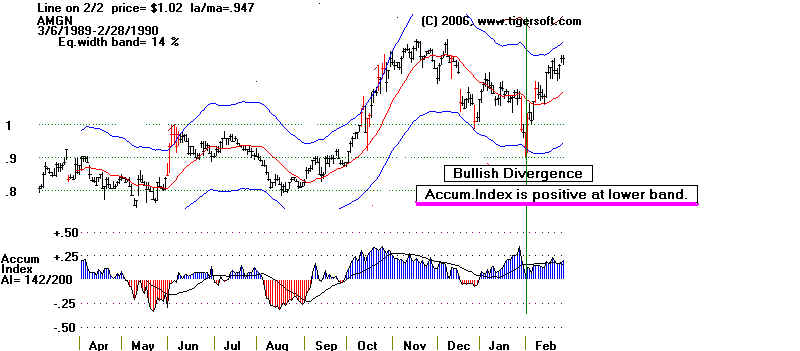
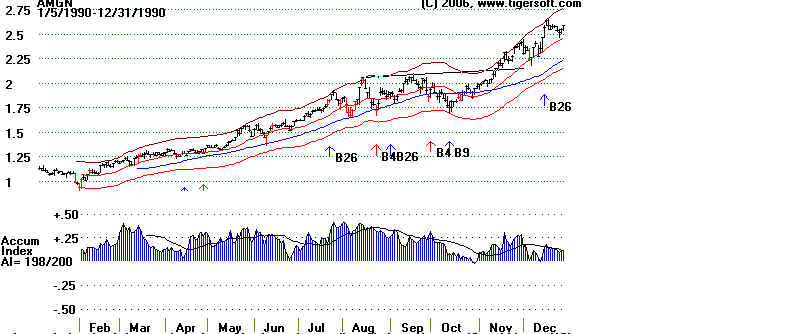
time AMGN stood out to us was back in late 1989. In the chart below you can see
the stock fell the lower band with
the Accumulation Index still in positive territory. This often
indicates an intermediate term
bottom, just like in May when it hit the upper band with
the Accumulation Index in negative
territory and then declined for two months. The stock
at the time of the October 1989
bullish divergence was only .98, when adjusted for all the splits
since then.
AMGEN 1989-1990
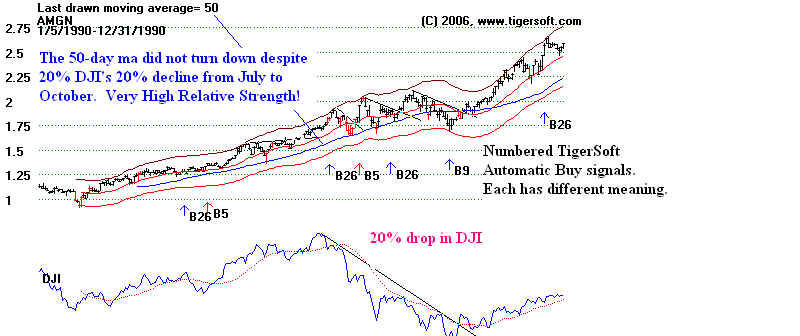
More obvious, in the next TigerSoft chart, below, you will want to note how AMGN
looked in 1990 as its big move began in
earnest. We can easily guess that doctors and
researchers must have been buying the
stock after seeing how efficacious its new test drugs,
Neupogen and Epogen, were. We can tell
this because the overall stock market was actually
quite weak in January, as the DJI fell
more than 10% until February and again from July to
October, as the DJI plunged almost 20%.
So, most investors would have been holding back.
When
the stock market falls like this, the strongest stocks stand out, like rocks in a cove
with the tide out. We use a simple
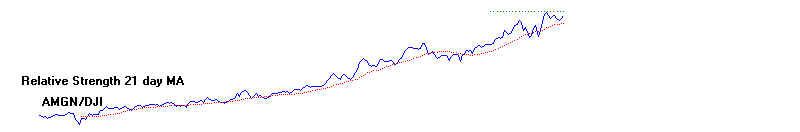
ratio of the stock and the DJI. That is shown below. And
we compare the stock's gain over a fixed
period of time with the DJI's gain over the same
duration. The second approach we
call "ITRS", for intermediate-term relative strength. The
"ITRS" for Amgen was positive
for the whole year. This is usually very bullish.
AMGEN and DJIA: 1990
Relative Strength Indicators
 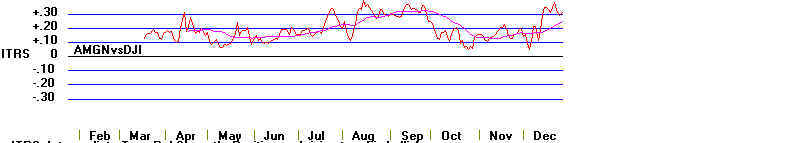
You can search the new highs list in a bear market and sometimes a stock like Amgen.
But it is better to be sure that the stock shows very positive readings from our
"Tiger Accumulation
Index". Look at the spectacularly bullish chart below. All but two days
produced a positive
Accumulation Index. The stock's decline to the lower band in October with the
Accumulation
Index positive gave us the "B9" signal shown above. The turning positive
by this indicator
after being negative for just a day is also a positive development. We see this in
October
and December. Tiger's Accumulation index was invented by William Schmidt in 1981.
AMGEN and Tiger's Accumulation Index: 1990
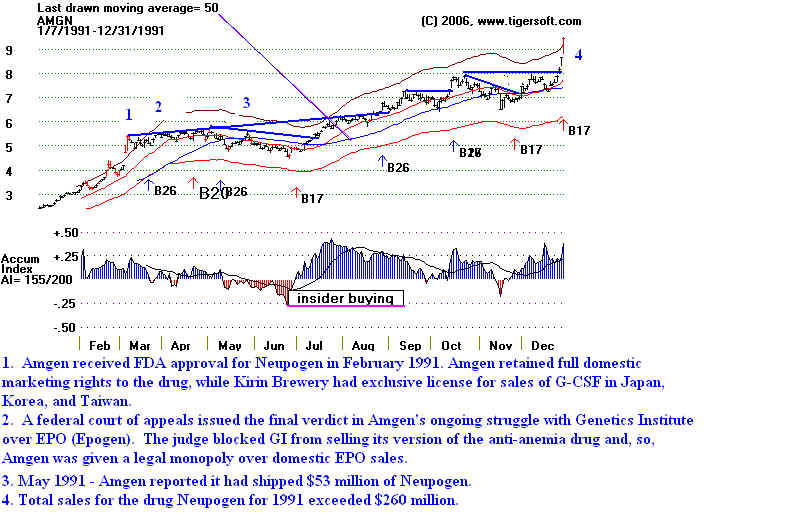
Now look at how AMGEN did with FDA approval for Neopogen in
February.
In May it won a court battle for exclusive rights to market
its drug Epogen.
Amgen and Tiger's Accumulation Index: 1991
Amgen 's 20 year chart is shown below
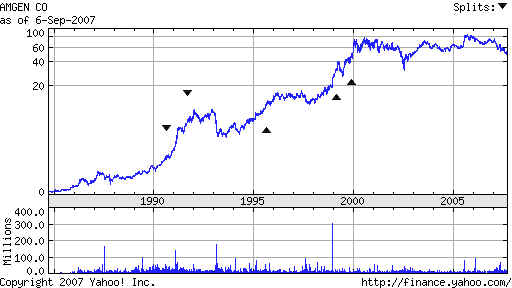
Amgen
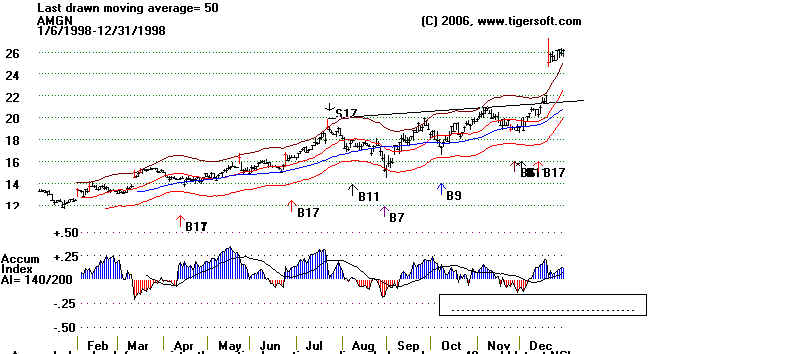
1998-2002: A Roller Coaster Ride with Ample Road Signs.
Note Amgen's steep advance
from 1998 to 2000. Its sales had by then passed
$3 billion. The FDA approved its ENBREL to treat moderately to
severely active
polyarticular-course juvenile rheumatoid arthritis. This
was unanimously recommended
for FDA approval on September 16, 1998. It was awarded FDA
approval on November
2, 1998. (Source:
http://arthritis.about.com/od/enbrel/a/enbrelrecommend.htm
and http://arthritis.about.com/od/enbrel/a/enbrelapproved.htm
)
Amgen and Tiger's Accumulation Index: 1998
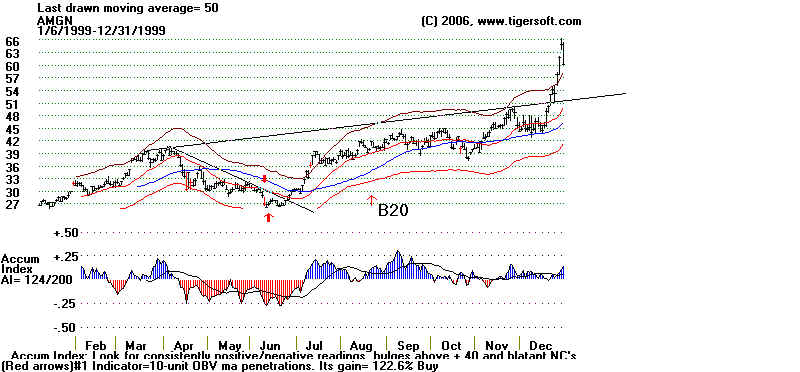
Amgen and Tiger's Accumulation Index: 1999
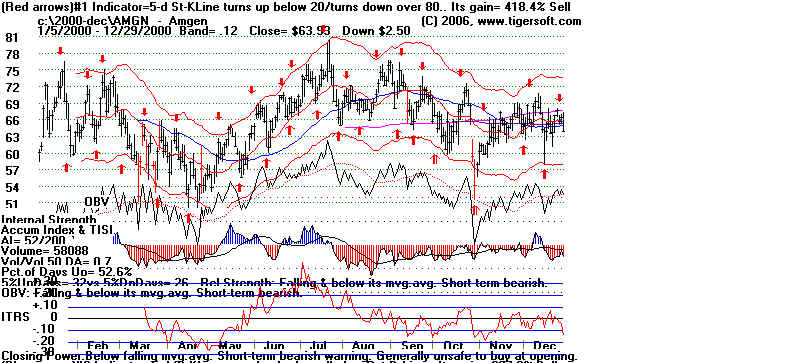
Amgen and Tiger's Accumulation Index: 2000
Note Steady Red Distribution
Amgen and Tiger's Accumulation Index: 2001
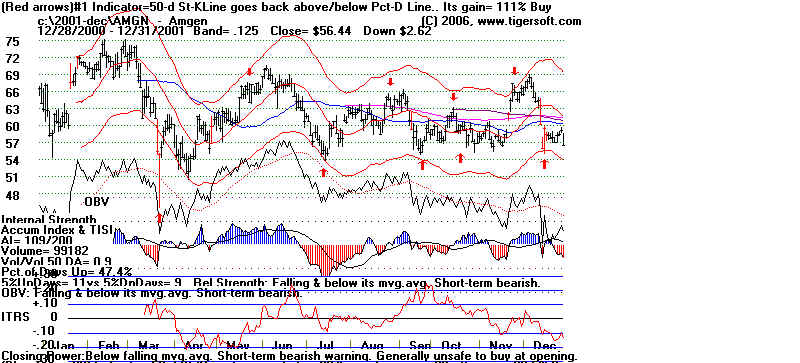
Amgen and Tiger's Accumulation Index: 2002
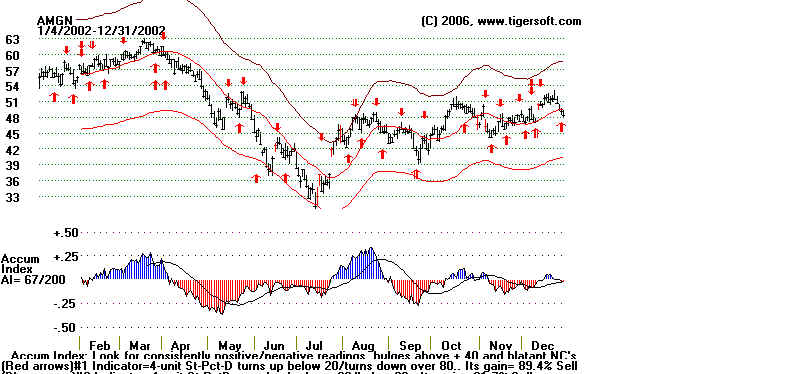
Amgen's Story
--- See http://www.answers.com/topic/amgen-inc?cat=biz-fin
The not-so-pretty picture of Amgen Lobbying
Thursday September 6, 3:42 pm ET
http://www.fool.com/investing/high-growth/2007/09/06/amgens-congressional-helpers.aspx
http://biz.yahoo.com/ap/070906/amgen_lobbying.html?.v=1 |
Amgen Paid Firm $480,000 to Lobby the Federal
Government
The Associated Press reported yesterday that the Senate
passed a resolution calling for the reversal of strict
reimbursement rules by the Centers for Medicare and Medicaid Services (CMMS) on Amgen's
lead drugs.
It has been a long year for Amgen and its two anemia-treating compounds, Epogen and
Aranesp. First, a Danish
study came out showing Aranesp did not improve quality of life or reduce the risk of blood
transfusions for those
with noncancer-related anemia. This put a serious dent in Amgen's plans to expand the use
of Aranesp, and sales
of the drug fell 10% year over year in the second quarter.
Shortly thereafter, the FDA slapped a stronger black
box safety warning on Amgen's two anemia compounds as
well as Johnson and Johnson's (NYSE: JNJ) anemia-treating Procrit.
The final nail in Amgen's erythropoiesis-
stimulating agents (ESA) growth plans came in July when the CMMS enacted stricter
reimbursement rules for
the ESA drug class that would curtail some usage in patients reliant on these government
insurance programs.
Amgen has argued that the CMMS reimbursement rules are unduly strict and don't follow
clinical practice guidelines
from leading doctors groups such as the American Society of Hematology. The CMMS and Amgen
have been going
back and forth on reimbursement rules for the ESAs for years.
Together, Aranesp and Epogen accounted for 44% of Amgen's sales in the latest quarter,
but times have been
tough for Amgen's top two compounds. In Europe, no fewer than three new competing agents
will be muscling these
drugs for market share by the end of next year, including Shire's (Nasdaq: SHPGY)
Dynepo, Novartis' (NYSE: NVS)
biosimilar
Epogen and Roche's Micera.
Amgen will get another chance to argue its case to the FDA about the risks and benefits
of its ESA drugs next week
during an FDA advisory committee meeting. The agency will take the recommendations from
this meeting into
consideration in developing new treatment guidelines for the ESAs, so Amgen could be in
for one more kick
in the tail this year.
WASHINGTON (AP) -- Drug maker Amgen Inc. paid Covington & Burling
LLP $480,000 in the first half of 2007
to lobby the federal government, according to a recent disclosure form.
The firm lobbied Congress on unspecified policy matters, according to the form posted
online Aug. 13 by the
Senate's public records office. Under a federal law enacted in 1995, lobbyists are
required to disclose activities that
could influence members of the executive and legislative branches. They must register with
Congress within 45 days
of being hired or engaging in lobbying.
|
|
