Short Squueze in DNDN Sends Stock Up 500% in Two Weeks!.
and
Does FDA Really Care about Terminal
Prostate Cancer Patients?
Dendreon (DNDN) 2006-2007
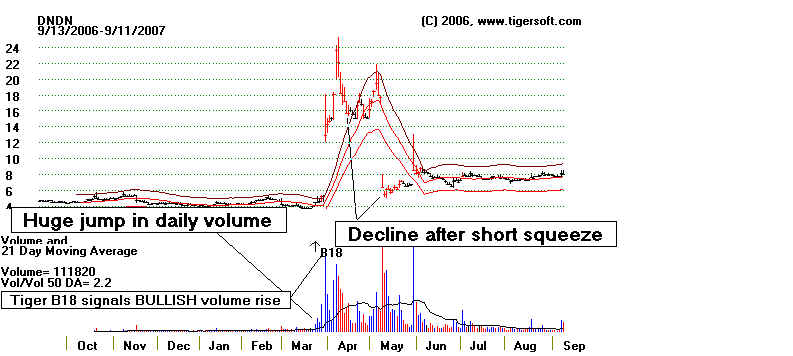
There's an important lesson here. Don't short a stock
that has too high a short interest.
And if unexpected good news should
befall the company, buy the stock aggressively. The
stock will go up, as the shorts
rush to cover.
Take the case of Dendreon (DNDN). In late March, just before its
breathtaking
ascent from $5 to a high of $25 in
7 trading days, its anti-cancer drug, a vacine named
Provenge, was considered by
an FDA advisory panel. Such panels routinely provide guidance
that the FDA usually accepts or
considers very closely in deciding whether or not to give a
drug final approval.
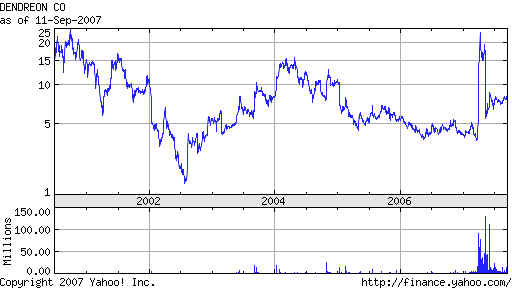
As you can see in the 7 year chart at the bottom of this page, DNDN had been steadily
falling since early 2004, when it
was 15. Reports had come out that its Provenge, which is used
to treat prostate cancer that does
not respond to hormone therapy, was not reaching its primary
"end-points" in the
trials for slowing the progress of the disease. As a result, it was widely
believed that the Advisory Panel would
recommend against the drug on grounds of unproven
efficacy as well as safety. And the
stock was heavily shorted. In March, 26.4 million shares
had been sold short. This was 11
times the average trading volume and an amzing 34.7% of all the
shares outstanding. Clearly many of
the shares sold short were "naked" short sales, where the
stock was never even borrowed when it was
sold. (The Securities and Exchange Commission
usually does little more than shake its
finger at the accused brokerages when such cases are
pointed out to them!)
The monthly short interest figures published by the NASDAQ.
Settlement
Date |
Short
Interest |
Avg Daily
Share Volume |
Days
to Cover |
| Aug. 15, 2007 |
36,029,103 |
4,134,767 |
8.71 |
| Jul. 13, 2007 |
42,339,655 |
8,288,430 |
5.11 |
| Jun. 15, 2007 |
40,847,280 |
20,081,558 |
2.03 |
| May 15, 2007 |
41,662,926 |
32,227,947 |
1.29 |
| Apr. 13, 2007 |
33,901,959 |
29,283,869 |
1.16 |
| Mar. 15, 2007 |
26,419,737 |
2,389,099 |
11.06 |
| Feb. 15, 2007 |
20,306,278 |
1,537,648 |
13.21 |
| Jan. 12, 2007 |
16,799,777 |
1,392,811 |
12.06 |
| Dec. 15, 2006 |
13,055,168 |
1,605,236 |
8.13 |
| Nov. 15, 2006 |
12,425,374 |
1,323,045 |
9.39 |
| Oct. 13, 2006 |
12,323,167 |
513,946 |
23.98 |
| Sep. 15, 2006 |
12,565,560 |
602,930 |
20.84 |
The Bullish Surprise.
To the surprise of all the shorts, though the drug did not
meet the goals set out
in the original study, the average survival time of patients on Provenge was increased by
3 to 5 months. Moreover, the mode of treatment was far less destructive of a
patient's final
days than the alternative, radiation therapy. /The FDA Advisory Panel voted
unanimously
that the drug was safe, as it was grown from the patient's own cells, and voted by 13-4
that
it was "effective". Word got out the day before the announcement.
On March 28th, trading
volume more than tripled from the day before and the stock rose from 4,612 to 5.22.
On the day
of the panel's meeting, the NASDAQ halted trading.. On the next day, March 30th, the
stock
tripled and opened at 17.92. It closed at 12.93. The volume that day was
925,478 shares,
a
9-fold increase from what it had been the day before the announcement. It rose each
of the
next
5 days and hit 25.25 on the sixth. It came very close to its all-time high.
The rise was
all
short squeeze.
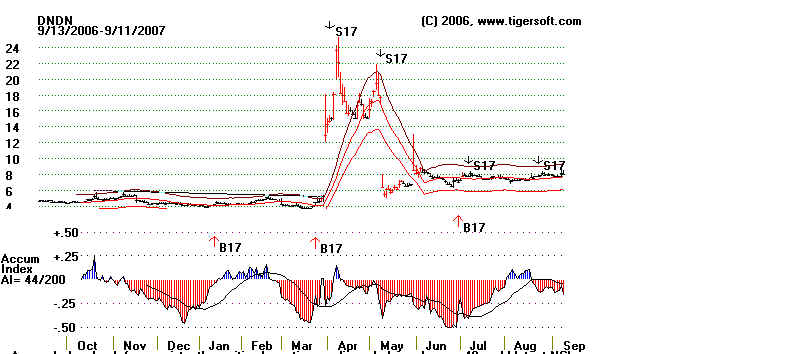
What's The Lesson Here.
Buy aggressively stocks that have unexpectely very good news
when the short
positions are so high. Watch the news in very high volume stocks, if you are a day
trader or even a short-term trader. And when you look at a Tiger chart, know
that
insiders can be wrong. The Tiger Accumulation chart shows steady red distribution,
reflecting heavy shorting. Seldom are the insider this wrong, but they can be very
wrong, in the short- term. Below is the Tiger Accumulation chart. In
fairness to it,
users know that sudden spikes of Accumulation are important. Tiger charts represent
these as Buy B17 and Sell S17 signals. Use these when the closing price is within
the bands. With this understood, these signals excel.
Back to Earth: Does The FDA Care?
DNDN's stock quickly fell back once the short squeeze was
over. And, as
if to prove the insiders were right all along to short the stock, on May 8th the FDA
disregarded its Panel's advise and raised new questions about the new vacine's
efficacy that will probably not be answered until 2010! So, ultimately, the cynical
insider-short sellers will probably be proven right. They understood how
bureaucratic
the FDA is. They understood how much more concerned the FDA is in protecting
itself from criticism it is than in alleviating pain and suffering among a class of
patients
that is presently quite doomed!
g
 |
Dendreon Corp.
3005 First Avenue
Seattle, WA 98121
United States - Map
Phone: 206-256-4545
Fax: 206-256-0571
Web Site: http://www.dendreon.com
DNDN engages in the discovery, development, and commercialization of therapeutics that
harness the immune system
to fight cancer. Its product portfolio includes active cellular immunotherapy, monoclonal
antibody, and small molecule
product candidates to treat various cancers. The company's product candidates include
Provenge (sipuleucel-T),
an active cellular immunotherapy that is in FDA priority review status for the treatment
of asymptomatic, metastatic,
androgen-independent prostate cancer. See also http://finance.yahoo.com/q/pr?s=DNDN |
Did The FDA Make A Mistake?
Q&A: Dendreon’s Vaccine for Prostate Cancer
Patients

An FDA advisory committee yesterday voted 13-4 in favor of Provenge, an experimental
treatment for advanced
prostate cancer. The vote was hotly anticipated by cancer patients and investors in
Dendreon, the Seattle-based
biotech company whose future is pegged to Provenge. Shares in the company rose more than
100% today. Provenge
commands special attention because it’s a vaccine, derived from a patient’s own
cells, that mobilizes the immune system
to attack cancer.
But what would Provenge mean for men with prostate cancer? To find out, we called Simon Hall,
director of the
Deane Prostate Health and Research center at the Mount Sinai School of Medicine in New
York, and an investigator
on a trial of Provenge. (Hall told the Health Blog he has no financial ties to Dendreon.)
Here are the highlights of our
conversation.
Q: If the FDA approves Provenge, how would it fit in with current treatment?
A: The vast majority of patients are diagnosed with localized disease, which is treated
with surgery or radiation.
About a third of those patients will have a recurrence — if they live long enough,
they’ll have metastatic disease and
have hormone treatment. For the vast majority of those patients, that will buy them
several years where their disease
is under control. But then the cells will adapt and start to grow anyway. I would estimate
between 29,000 and 40,000
men are in this boat. The only treatment that has ever been shown to have any advantage
for these patients was taxotere
[a chemotherapy drug from Sanofi-Aventis], shown to extend life by 3 months. But the
bottom line is patients don’t
want chemotherapy. The reality will be that when the patients get to this point,
they’re probably going to do the vaccine.
I think there will be a huge demand. I’ve had a lot of my own patients calling me and
saying, “Can I get the vaccine?”
Q: Any idea how much Provenge will cost? Will the price influence who gets it?
A: It’s going to be a very expensive, boutique-type agent. Different from drugs or
even vaccines that are in the pipeline,
this one is custom made for each individual patient with their own cells. A lot of times
we use drugs off label; I don’t
think it will happen in this case because of the expense. The rumor is it will cost
$20,000 per infusion. And the way
the trials have been done, it’s been three infusions per patient.
Q: The clinical studies failed to meet their goals, but suggested the vaccine
prolongs survival for four months.
Is that significant?
A: You set up a study and you say my primary endpoint is time to progression, and my
secondary endpoint is delaying
time to pain. They failed on that. They were disappointed, and I’m sure they had
statisticians come in and say, “How
can we carve up the data and get something out of the study? And they were like,
“Holy cow, at 36 months, only 10%
of those on placebo were still alive, but you’ve got a third of those on Provenge
still alive.”
Q: Do you think the FDA will follow the committee’s advice?
It’s hard to guess. Would they approve a drug based on one very small study that was
sort of done backwards?
A committee looks at it and votes 13-4 to approve. I don’t know what they will do.
Will they approve?
Or will they say, “Listen, you’ve got this other big study that’s going on
and maybe we should wait a year and see
what that says.”
Q: What’s the bottom line for Provenge?
We need it. There’s little toxicity. If the larger study validates the first study,
or clinical experience validates it, it will be
a step forward for men with prostate cancer, and for this whole idea of using the immune
system to fight cancer.
(Source: http://blogs.wsj.com/health/2007/03/30/qa-dendreons-vaccine-for-prostate-cancer-patients/
)
|
.
|




